Blog
- Key Difference in the Brains of Super-Agers
- By Jason von Stietz, M.A.
- March 9, 2018
-

Getty Images Why do some people in their 90’s seem mentally sharp when others do not? Researchers at Northwestern University examined the brains and cognitive abilities of “super-agers.” Findings from autopsies showed that the brains of super-agers had a significantly thicker cortex than those of their average peers. The study was discussed in a recent article in MedicalXpress:
It's pretty extraordinary for people in their 80s and 90s to keep the same sharp memory as someone several decades younger, and now scientists are peeking into the brains of these "superagers" to uncover their secret.
The work is the flip side of the disappointing hunt for new drugs to fight or prevent Alzheimer's disease.
Instead, "why don't we figure out what it is we might need to do to maximize our memory?" said neuroscientist Emily Rogalski, who leads the SuperAging study at Northwestern University in Chicago.
Parts of the brain shrink with age, one of the reasons why most people experience a gradual slowing of at least some types of memory late in life, even if they avoid diseases like Alzheimer's.
But it turns out that superagers' brains aren't shrinking nearly as fast as their peers'. And autopsies of the first superagers to die during the study show they harbor a lot more of a special kind of nerve cell in a deep brain region that's important for attention, Rogalski told a recent meeting of the American Association for the Advancement of Science.
These elite elders are "more than just an oddity or a rarity," said neuroscientist Molly Wagster of the National Institute on Aging, which helps fund the research. "There's the potential for learning an enormous amount and applying it to the rest of us, and even to those who may be on a trajectory for some type of neurodegenerative disease."
What does it take to be a superager? A youthful brain in the body of someone 80 or older. Rogalski's team has given a battery of tests to more than 1,000 people who thought they'd qualify, and only about 5 percent pass. The key memory challenge: Listen to 15 unrelated words, and a half-hour later recall at least nine. That's the norm for 50-year-olds, but the average 80-year-old recalls five. Some superagers remember them all.
"It doesn't mean you're any smarter," stressed superager William "Bill" Gurolnick, who turns 87 next month and joined the study two years ago.
Nor can he credit protective genes: Gurolnick's father developed Alzheimer's in his 50s. He thinks his own stellar memory is bolstered by keeping busy. He bikes, and plays tennis and water volleyball. He stays social through regular lunches and meetings with a men's group he co-founded.
"Absolutely that's a critical factor about keeping your wits about you," exclaimed Gurolnick, fresh off his monthly gin game.
Rogalski's superagers tend to be extroverts and report strong social networks, but otherwise they come from all walks of life, making it hard to find a common trait for brain health. Some went to college, some didn't. Some have high IQs, some are average. She's studied people who've experienced enormous trauma, including a Holocaust survivor; fitness buffs and smokers; teetotalers and those who tout a nightly martini.
But deep in their brains is where she's finding compelling hints that somehow, superagers are more resilient against the ravages of time.
Early on, brain scans showed that a superager's cortex—an outer brain layer critical for memory and other key functions—is much thicker than normal for their age. It looks more like the cortex of healthy 50- and 60-year-olds.
It's not clear if they were born that way. But Rogalski's team found another possible explanation: A superager's cortex doesn't shrink as fast. Over 18 months, average 80-somethings experienced more than twice the rate of loss.
Another clue: Deeper in the brain, that attention region is larger in superagers, too. And inside, autopsies showed that brain region was packed with unusual large, spindly neurons—a special and little understood type called von Economo neurons thought to play a role in social processing and awareness.
The superagers had four to five times more of those neurons than the typical octogenarian, Rogalski said—more even than the average young adult.
The Northwestern study isn't the only attempt at unraveling long-lasting memory. At the University of California, Irvine, Dr. Claudia Kawas studies the oldest-old, people 90 and above. Some have Alzheimer's. Some have maintained excellent memory and some are in between.
About 40 percent of the oldest-old who showed no symptoms of dementia in life nonetheless have full-fledged signs of Alzheimer's disease in their brains at death, Kawas told the AAAS meeting.
Rogalski also found varying amounts of amyloid and tau, hallmark Alzheimer's proteins, in the brains of some superagers.
Now scientists are exploring how these people deflect damage. Maybe superagers have different pathways to brain health.
"They are living long and living well," Rogalski said. "Are there modifiable things we can think about today, in our everyday lives" to do the same?
View the original article here
- Comments (0)
- Exercise Recommended in Treatment of Mild Cognitive Impairment
- By Jason von Stietz, MA
- January 11, 2018
-

Getty Images According to the American Academy of Neurology, 6 percent of individuals in their 60’s suffer from mild cognitive impairment. Clinicians from the Mayo Clinic suggest that regular physical exercise totaling 150 minutes a week should be part of treatment to address symptoms related to mild cognitive impairment. This new guideline was discussed in recent article in Medical Xpress:
The recommendation is part of an updated guideline for mild cognitive impairment published in the Dec. 27 online issue of Neurology, the medical journal of the American Academy of Neurology.
"Regular physical exercise has long been shown to have heart health benefits, and now we can say exercise also may help improve memory for people with mild cognitive impairment," says Ronald Petersen, M.D., Ph.D., lead author, director of the Alzheimer's Disease Research Center, Mayo Clinic, and the Mayo Clinic Study of Aging. "What's good for your heart can be good for your brain." Dr. Petersen is the Cora Kanow Professor of Alzheimer's Disease Research.
Mild cognitive impairment is an intermediate stage between the expected cognitive decline of normal aging and the more serious decline of dementia. Symptoms can involve problems with memory, language, thinking and judgment that are greater than normal age-related changes.
Generally, these changes aren't severe enough to significantly interfere with day-to-day life and usual activities. However, mild cognitive impairment may increase the risk of later progressing to dementia caused by Alzheimer's disease or other neurological conditions. But some people with mild cognitive impairment never get worse, and a few eventually get better.
The academy's guideline authors developed the updated recommendations on mild cognitive impairment after reviewing all available studies. Six-month studies showed twice-weekly workouts may help people with mild cognitive impairment as part of an overall approach to managing their symptoms.
Dr. Petersen encourages people to do aerobic exercise: Walk briskly, jog, whatever you like to do, for 150 minutes a week—30 minutes, five times or 50 minutes, three times. The level of exertion should be enough to work up a bit of a sweat but doesn't need to be so rigorous that you can't hold a conversation. "Exercising might slow down the rate at which you would progress from mild cognitive impairment to dementia," he says.
Another guideline update says clinicians may recommend cognitive training for people with mild cognitive impairment. Cognitive training uses repetitive memory and reasoning exercises that may be computer-assisted or done in person individually or in small groups. There is weak evidence that cognitive training may improve measures of cognitive function, the guideline notes.
The guideline did not recommend dietary changes or medications. There are no drugs for mild cognitive impairment approved by the U.S. Food and Drug Administration.
More than 6 percent of people in their 60s have mild cognitive impairment across the globe, and the condition becomes more common with age, according to the American Academy of Neurology. More than 37 percent of people 85 and older have it.
With such prevalence, finding lifestyle factors that may slow down the rate of cognitive impairment can make a big difference to individuals and society, Dr. Petersen notes.
"We need not look at aging as a passive process; we can do something about the course of our aging," he says. "So if I'm destined to become cognitively impaired at age 72, I can exercise and push that back to 75 or 78. That's a big deal."
The guideline, endorsed by the Alzheimer's Association, updates a 2001 academy recommendation on mild cognitive impairment. Dr. Petersen was involved in the development of the first clinical trial for mild cognitive impairment and continues as a worldwide leader researching this stage of disease when symptoms possibly could be stopped or reversed.
View to the article here
- Comments (0)
- Brain Implant Boosts Short-Term and Working Memory
- By Jason von Stietz, MA
- November 30, 2017
-

Researchers from University of Southern California have used a brain implant to help the brain’s of study participants function more effectively. Electrodes were implanted into the brain’s of participants. Once the pattern of activity associated with optimal memory performance was determined, the electrode was used to stimulate the brain reinforcing the optimal pattern. Findings indicated the short-term memory was improved by 15% and working memory was improved by 25%. The study was discussed in a recent article in Futurism:
With everyone from Elon Musk to MIT to the U.S. Department of Defense researching brain implants, it seems only a matter of time before such devices are ready to help humans extend their natural capabilities. Now, a professor from the University of Southern California (USC) has demonstrated the use of a brain implant to improve the human memory, and the device could have major implications for the treatment of one of the U.S.’s deadliest diseases.
Dong Song is a research associate professor of biomedical engineering at USC, and he recently presented his findings on a “memory prosthesis” during a meeting of the Society for Neuroscience in Washington D.C. According to a New Scientist report, the device is the first to effectively improve the human memory.
To test his device, Song’s team enlisted the help of 20 volunteers who were having brain electrodes implanted for the treatment of epilepsy.
Once implanted in the volunteers, Song’s device could collect data on their brain activity during tests designed to stimulate either short-term memory or working memory. The researchers then determined the pattern associated with optimal memory performance and used the device’s electrodes to stimulate the brain following that pattern during later tests.
Based on their research, such stimulation improved short-term memory by roughly 15 percent and working memory by about 25 percent. When the researchers stimulated the brain randomly, performance worsened.
As Song told New Scientist, “We are writing the neural code to enhance memory function. This has never been done before.”
A GROWING PROBLEM
While a better memory could be useful for students cramming for tests or those of us with trouble remembering names, it could be absolutely life-changing for people affected by dementia and Alzheimer’s.
As Bill Gates noted when announcing plans to invest $100 million of his own money into dementia and Alzheimer’s research, the disease is a multi-level problem that’s positioned to get even worse.
Age is the greatest risk factor for Alzheimer’s, according to the Alzheimer’s Association, with the vast majority of sufferers over the age of 65. With advances in medicine and healthcare continuously increasing how long we live, that segment of the population is growing dramatically, and by 2030, 20 percent of U.S. citizens are expected to be older than 65.
This increase in the number of potential dementia sufferers can be costly in both a financial and emotional sense. In 2016, the total cost of healthcare and long-term care for those suffering from dementia and Alzheimer’s disease was an estimated $236 billion, and according to the Alzheimer’s Association, the more severe a person’s cognitive impairment, the higher the rates of depression in their familial caregivers.
Of course, further testing is required before Song’s device could be approved as a treatment for dementia or Alzheimer’s, but if it is able to help those patients regain even part of their lost memory function, the impact would be felt not only by the patients themselves, but their families and even the economy at large.
Read the article Here
- Comments (0)
- Study Identifies Biomarkers for Alzheimer's Disease
- By Jason von Stietz, M.A.
- August 15, 2016
-
.jpg)
Getty Images Researchers at the University of Wisconsin Madison have identified biomarkers helpful in predicting the development of Alzheimer’s disease. The researchers analyzed the data of 175, which included brain scans, measures of cognitive abilities, and genotyping. The study was discussed in a recent article in NeuroscientistNews:
This approach – which statistically analyzes a panel of biomarkers – could help identify people most likely to benefit from drugs or other interventions to slow the progress of the disease. The study was published in the August edition of the journal Brain.
"The Alzheimer's Association estimates that if we had a prevention that merely pushed back the clinical symptoms of the disease by five years, it would almost cut in half the number of people projected to develop dementia,'' says Annie Racine, a doctoral candidate and the study's lead author. "That translates into millions of lives and billions of dollars saved."
Dr. Sterling Johnson, the study's senior author, says that while brain changes – such as the buildup of beta-amyloid plaques and tangles of another substance called tau – are markers of the disease, not everyone with these brain changes develops Alzheimer's symptoms.
"Until now, we haven't had a great way to use the biomarkers to predict who was going to develop clinical symptoms of the disease,'' Johnson says. "Although the new algorithm isn't perfect, now we can say with greater certainty who is at increased risk and more likely to decline."
The research team recruited 175 older adults at risk for Alzheimer's disease, and used statistical algorithms to categorize them into four clusters based on different patterns and profiles of pathology in their brains. Then, the researchers analyzed cognitive data from the participants to investigate whether these cluster groups differed on their cognitive abilities measured over as many as 10 years.
As it turns out, the biomarker panels were predictive of cognitive decline over time. One cluster in particular stood out. The group that had a biomarker profile consistent with Alzheimer's – abnormal levels of tau and beta-amyloid in their cerebrospinal fluid – showed the steepest decline on cognitive tests of memory and language over the 10 years of testing. About two-thirds of the 22 people sorted into this group were also positive for the APOE4 gene—the greatest known risk factor for sporadic Alzheimer's disease—compared with about one-third in the other clusters.
At the other end of the spectrum, the largest group, 76 people, were sorted into a cluster that appears to be made up of healthy aging individuals. They showed normal levels on the five biomarkers and did not decline cognitively over time.
In between, there were two clusters that weren't classified as Alzheimer's but who don't seem to be aging normally either. A group of 32 people showed signs of mixed Alzheimer's and vascular dementia. They had some of the amyloid changes in their cerebrospinal fluid, but also showed lesions in their brains' white matter, which indicate scarring from small ischemic lesions which can be thought of as minor silent strokes.
The other cluster of 45 people had signs of brain atrophy, with brain imaging showing that the hippocampus, the brain's memory center, was significantly smaller than the other groups. The authors speculate this group could have intrinsic brain vulnerability or could be affected by some other process that differentiates them from healthy aging. Both the in-between clusters showed non-specific decline on a test of global cognitive functioning, which further differentiates them from the healthy aging cluster.
The study participants came from a group of more than 1,800 people enrolled in two studies – the Wisconsin Alzheimer's Disease Research Center (WADRC) study and the Wisconsin Registry for Alzheimer's Prevention (WRAP). Both groups are enriched for people at risk for getting Alzheimer's because about ¾ of participants have a parent with the disease.
"This study shows that just having a family history doesn't mean you are going to get this disease," Johnson says. "Some of the people in our studies are on a trajectory for Alzheimer's, but more of them are aging normally, and some are on track to have a different type of brain disease." A comprehensive panel of biomarkers – such as the one evaluated in this study – could help to predict those variable paths, paving the way for early interventions to stop or slow the disease.
The authors of the study are affiliated with the WADRC, the Wisconsin Alzheimer's Institute, the Institute on Aging, the Waisman Center, and the Neuroscience and Public Policy program, all at UW-Madison; and the Geriatrics Research Education and Clinical Center at the William S. Middleton Veterans Hospital.
Read the original Here
- Comments (0)
- Strategy-Based Training Might Help Mild Cognitive Impairment
- By Jason von Stietz, M.A.
- June 26, 2016
-
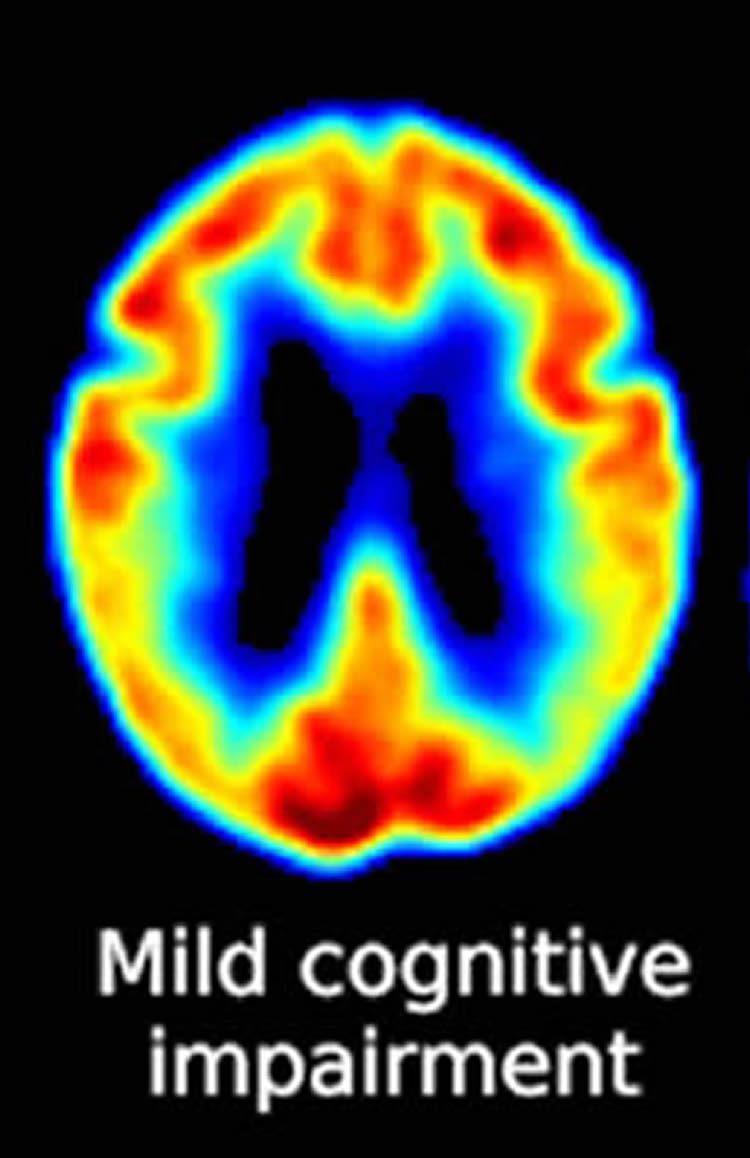
Photo Credit: Neuroscience News Making sense of everyday spoken and written language is an ongoing daily challenge for individuals with mild cognitive impairment, who are in a preclinical stage of Alzheimer’s disease. Researchers at the University of Texas at Dallas and the University of Illinois at Urbana Champagne investigated the usefulness of strategy-based reasoning training in improving cognitive ability. The study was discussed in a recent article in Neuroscience News:
“Changes in memory associated with MCI are often disconcerting, but cognitive challenges such as lapses in sound decision-making and judgment can have potentially worse consequences,” said Dr. Sandra Bond Chapman, founder and chief director at the Center for BrainHealth and Dee Wyly Distinguished University Professor in the School of Behavioral and Brain Sciences. “Interventions that mitigate cognitive deterioration without causing side effects may provide an additive, safe option for individuals who are worried about brain and memory changes.”
For the study, 50 adults ages 54-94 with amnestic MCI were randomly assigned to either a strategy-based, gist reasoning training group or a new-learning control group. Each group received two hour-long training sessions each week. The gist reasoning group received and practiced strategies on how to absorb and understand complex information, and the new-learning group used an educational approach to teach and discuss facts about how the brain works and what factors influence brain health.
Strategies in the gist reasoning training group focused on higher-level brain functions such as strategic attention — the ability to block out distractions and irrelevant details and focus on what is important; integrated reasoning — the ability to synthesize new information by extracting a memorable essence, pearl of wisdom, or take-home message; and innovation — the ability to appreciate diverse perspectives, derive multiple interpretations and generate new ideas to solve problems.
Pre- and post-training assessments measured changes in cognitive functions between the two groups. The gist reasoning group improved in executive function (i.e., strategic attention to recall more important items over less-important ones) and memory span (i.e., how many details a person can hold in their memory after one exposure, such as a phone number). The new learning group improved in detail memory (i.e., a person’s ability to remember details from contextual information). Those in the gist reasoning group also saw gains in concept abstraction, or an individual’s ability to process and abstract relationships to find similarities (e.g., how are a car and a train alike).
“Our findings support the potential benefit of gist reasoning training as a way to strengthen cognitive domains that have implications for everyday functioning in individuals with MCI,” said Dr. Raksha Mudar, study lead author and assistant professor at the University of Illinois at Urbana-Champaign. “We are excited about these preliminary findings, and we plan to study the long-term benefits and the brain changes associated with gist reasoning training in subsequent clinical trials.”
“Extracting sense from written and spoken language is a key daily life challenge for anyone with brain impairment, and this study shows that gist reasoning training significantly enhances this ability in a group of MCI patients,” said Dr. Ian Robertson, T. Boone Pickens Distinguished Scientist at the Center for BrainHealth and co-director of The Global Brain Health Initiative. “This is the first study of its kind and represents a very important development in the growing field of cognitive training for age-related cognitive and neurodegenerative disorders.”
“Findings from this study, in addition to our previous Alzheimer’s research, support the potential for cognitive training, and specifically gist reasoning training, to impact cognitive function for those with MCI,” said Audette Rackley, head of special programs at the Center for BrainHealth. “We hope studies like ours will aid in the development of multidimensional treatment options for an ever-growing number of people with concerns about memory in the absence of dementia.”
Read the original article Here
- Comments (0)
- Anticholinergics Linked to Changes in Brain and Cognitive Impairment
- By Jason von Stietz, M.A.
- April 26, 2016
-

Getty Images Are over the counter drugs completely safe? Researchers at Indiana University School of Medicine used positron emission tests (PET) to study the relationship between anticholinergic drugs and cognitive decline. Anticholinergics are commonly used as sleep aids as wells as treatments for hypertension and cardiovascular disease. Findings linked the use of anticholinergics to physical changes in the brain and cognitive impairment. The study was discussed in a recent article in Medical Xpress:
Older adults might want to avoid a using class of drugs commonly used in over-the-counter products such as nighttime cold medicines due to their links to cognitive impairment, a research team led by scientists at Indiana University School of Medicine has recommended.
Using brain imaging techniques, the researchers found lower metabolism and reduced brain sizes among study participants taking the drugs known to have an anticholinergic effect, meaning they block acetylcholine, a nervous system neurotransmitter.
Previous research found a link between between the anticholinergic drugs and cognitive impairment and increased risk of dementia. The new paper published in the journal JAMA Neurology, is believed to be the first to study the potential underlying biology of those clinical links using neuroimaging measurements of brain metabolism and atrophy.
"These findings provide us with a much better understanding of how this class of drugs may act upon the brain in ways that might raise the risk of cognitive impairment and dementia," said Shannon Risacher, Ph.D., assistant professor of radiology and imaging sciences, first author of the paper, "Association Between Anticholinergic Medication Use and Cognition, Brain Metabolism, and Brain Atrophy in Cognitively Normal Older Adults."
"Given all the research evidence, physicians might want to consider alternatives to anticholinergic medications if available when working with their older patients," Dr. Risacher said.
Drugs with anticholinergic effects are sold over the counter and by prescription as sleep aids and for many chronic diseases including hypertension, cardiovascular disease, and chronic obstructive pulmonary disease.
A list of anticholinergic drugs and their potential impact is athttp://www.agingbraincare.org/uploads/products/ACB_scale_-_legal_size.pdf.
Scientists have linked anticholinergic drugs cognitive problems among older adults for at least 10 years. A 2013 study by scientists at the IU Center for Aging Research and the Regenstrief Institute found that drugs with a strong anticholinergic effect cause cognitive problems when taken continuously for as few as 60 days. Drugs with a weaker effect could cause impairment within 90 days.
The current research project involved 451 participants, 60 of whom were taking at least one medication with medium or high anticholinergic activity. The participants were drawn from a national Alzheimer's research project—the Alzheimer's Disease Neuroimaging Initiative—and the Indiana Memory and Aging Study.
To identify possible physical and physiological changes that could be associated with the reported effects, researchers assessed the results of memory and other cognitive tests, positron emission tests (PET) measuring brain metabolism, and magnetic resonance imaging (MRI) scans for brain structure.
The cognitive tests revealed that patients taking anticholinergic drugs performed worse than older adults not taking the drugs on short-term memory and some tests of executive function, which cover a range of activities such as verbal reasoning, planning, and problem solving.
Anticholinergic drug users also showed lower levels of glucose metabolism—a biomarker for brain activity—in both the overall brain and in the hippocampus, a region of the brain associated with memory and which has been identified as affected early by Alzheimer's disease.
The researchers also found significant links between brain structure revealed by the MRI scans and anticholinergic drug use, with the participants using anticholinergic drugs having reduced brain volume and larger ventricles, the cavities inside the brain.
"These findings might give us clues to the biological basis for the cognitive problems associated with anticholinergic drugs, but additional studies are needed if we are to truly understand the mechanisms involved," Dr. Risacher said.
Read the original article Here
- Comments (0)
- Brain's Stress Circuitry Involved in Alzheimer's Disease Treatment
- By Jason von Stietz, M.A.
- November 27, 2015
-
Getty Images Researchers at the University of San Diego School of Medicine investigated the use of a small molecule drug in preventing and treating Alzheimer’s disease in mice. Researchers found that the drug significantly reduces activity of the stress circuitry in the brains of the mice and resulted in the prevention of neurodegeneration and cognitive impairment. The study was discussed in a recent article of NeuroScientistNews:
The findings are described in the current online issue of the journal Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association.
The results underscore the complexity and diversity of AD, whose causes appear to be a mix of genetic, lifestyle and environmental factors. Previous research has shown a link between the brain’s stress signaling pathways and AD. Specifically, the release of a stress-coping hormone called corticotropin-releasing factor (CRF), which is widely found in the brain and acts as a neurotransmitter/neuromodulator, is dysregulated in AD and is associated with impaired cognition and with detrimental changes in tau protein and increased production of amyloid-beta—protein fragments that clump together and trigger the neurodegeneration characteristic of AD.
“Our work and that of our colleagues on stress and CRF have been mechanistically implicated in Alzheimer’s disease, but agents that impact CRF signaling have not been carefully tested for therapeutic efficacy or long-term safety in animal models,” said the study’s principal investigator and corresponding author Robert Rissman, PhD, assistant professor in the Department of Neurosciences and Biomarker Core Director for the Alzheimer’s Disease Cooperative Study (ADCS).
“The novelty of this study is two-fold: We used a preclinical prevention paradigm of a CRF-antagonist (a drug that blocks the CRF receptor in brain cells) called R121919 in a well-established AD model – and we did so in a way that draws upon our experience in human trials. We found that R121919 antagonism of CRF-receptor-1 prevented onset of cognitive impairment and synaptic/dendritic loss in AD mice.”
In other words, the researchers determined that modulating the mouse brain’s stress circuitry (without actually changing the normal response) mitigated generation and accumulation of amyloid plaques widely attributed with causing neuronal damage and death. As a consequence, behavioral indicators of AD were prevented and cellular damage was reduced. The mice began treatment at 30-days-old – before any pathological or cognitive signs of AD were present – and continued until six months of age.
One particular challenge, Rissman noted, is limiting exposure of the drug to the brain so that it does not impact the body’s ability to response to stress. “This can be accomplished because one advantage of these types of small molecule drugs is that they readily cross the blood-brain barrier and actually prefer to act in the brain,” Rissman said. Drugs like R121919 were originally designed to treat generalized anxiety disorder, irritable bowel syndrome and other diseases, but failed to be effective in treating those disorders.
“Rissman’s prior work demonstrated that CRF and its receptors are integrally involved in changes in another AD hallmark, tau phosphorylation,” said William Mobley, MD, PhD, chair of the Department of Neurosciences and interim co-director of the Alzheimer’s Disease Cooperative Study at UC San Diego. “This new study extends those original mechanistic findings to the amyloid pathway and preservation of cellular and synaptic connections. Work like this is an excellent example of UC San Diego’s bench-to-bedside legacy, whereby we can quickly move our basic science findings into the clinic for testing,” said Mobley.
Rissman said R121919 was well-tolerated by AD mice (no significant adverse effects) and deemed safe, suggesting CRF-antagonism is a viable, disease-modifying therapy for AD. Rissman noted that repurposing R121919 for human use was likely not possible at this point. He and colleagues are collaborating with the Sanford Burnham Prebys Medical Discovery Institute to design new assays to discover the next generation of CRF receptor-1 antagonists for testing in early phase human safety trials.
“More work remains to be done, but this is the kind of basic research that is fundamental to ultimately finding a way to cure – or even prevent – Alzheimer’s disease,” said David Brenner, MD, vice chancellor, UC San Diego Health Sciences and dean of UC San Diego School of Medicine. “These findings by Dr. Rissman and his colleagues at UC San Diego and at collaborating institutions on the Mesa suggest we are on the cusp of creating truly effective therapies.”
Read the full article Here
- Comments (3)
- Possible Early Indication of Alzheimer's Found
- By Jason von Stietz
- March 6, 2015
-

Photo Credit: Getty Images The earliest signs of Alzheimer’s disease may have been found in a recent study. Researchers from Northwestern University examined the brains of those with Alzheimer’s and the brains of healthy participants. Findings suggested that amyloid protein, which are indicative of the disease, were found in healthy participants in their early 20’s. Cassie Shortsleeve discussed the study and possible protective strategies in a recent article in Yahoo Health:
Alzheimer’s disease may not be just for grandparents to worry about: Groundbreaking new research from Northwestern University has found that amyloid protein — a hallmark of the devastating disease — starts accumulating in brain neurons of people as young as 20 years old.
Scientists believe this is the first time that such changes have been noted in human brains so young. In the study, lead researcher Changiz Geula and his team from the Cognitive Neurology and Alzheimer’s Disease Center at Northwestern University’s Feinberg School of Medicine and team analyzed neurons from the brains of 13 “normal” young people ages 20 to 66; 16 people ages 70 to 99 without dementia; and 21 people with Alzheimer’s, ages 60 to 95.
“It was the age that really that surprised us,” Geula tells Yahoo! Health. “In the young adults, we already see accumulation of amyloids.” This becomes worrisome when amyloid clumps grow in size and abundance. The difference between old and young in this study: While the amyloids themselves were present in younger people — and clumps were present as well — there was more clumping in the aging and Alzheimer’s population, says Geula.
“What this means is these neurons are susceptible to accumulate at a young age, but that the clumping really occurs in aging. During life, the substance needed to make clumps is available. And if you have susceptibility to form clumps, this could worsen.”
So what does this mean for you? First, know this: “In this study, we didn’t have a huge number of brains,” says Geula. “And this doesn’t mean that because young people have a measure of amyloids that everyone is going to get Alzheimer’s. It’s not an alarm.”
But there are susceptibility factors (that science knows a lot about) — and protective factors (that science doesn’t know as much about) — when it comes to Alzheimer’s, Geula says. “We have known for a while that if we want effective therapy for Alzheimer’s, we have to start early. What these findings suggest is the earlier the better.”
So start today and protect yourself — and your brain —with these techniques, no matter your age.
1. Kick bad habits—stat. “We know that general cognitive aging and Alzheimer’s aging can be enhanced by many things — most importantly, general health,” says Geula. In fact, many health factors — diabetes, heart disease, pulmonary function, and obesity — can increase your risk of the disease.
2. Clean up your diet. Healthy habits like eating a Mediterranean diet (rich in nuts, greens, whole grains, fruits and veggies, poultry, and olive oil) have been linked to better cognitive function and a lower risk of Alzheimer’s.
3. Work it out. Exercise has been shown to enhance cognitive abilities in everyone, including the old and even the Alzheimer’s population. “Some animal studies show that the total amount of amyloid can be reduced in animals through enhanced exercise and enriched environment,” says Geula. And not only can exercise keep Alzheimer’s and dementia at bay, some research suggests it can even turn it around. When people with mild cognitive impairment walked on a treadmill for 30 minutes a day, four days a week for 12 weeks, people improved their neural efficiency using fewer mental resources to perform the same task.
4. Better your brain. Mental exercise — keeping your mind engaged and challenged through a variety of exercises — can also help. Research suggests that people who play cards, do crossword puzzles, and challenge themselves mentally on a regular basis are at a lower risk for developing the disease than those who don’t. And thinking on the bright side actually does matter: Negative thoughts can hinder your brain’s ability to think straight and form memories, according to research from King’s College in London.Read the original article Here
- Comments (0)
- A Second Language May Help Sustain the Brain
- By Jason von Stietz
- June 20, 2014
-

Photo Credit: Getty Images Could learning more than one language help you to think more clearly in later life? A researcher at the University of Edinburgh Center for Cognitive Aging and Cognitive Epidemiology examined IQ scores across the lifespan of those who spoke only one language and those who learned more than one. Those were bilingual performed better on cognitive tests than those who were monolingual, even if they had not scored better on tests decades earlier. Furthermore, bilingual study participants who suffered dementia began experiencing symptoms four to 5 years later than those who were monolingual. A recent article in The Washington Post discussed the University of Edinburgh researcher's study:
In his new study, older bilinguals performed better on cognitive tests than monolinguals, even when they had not scored better on intelligence testing decades earlier. That means that, at least in part, learning another language does predict brain health in old age, Bak said.
“Probably the causality is going in both directions, but we showed that there is certainly an effect of bilingualism that cannot be explained by previous differences,” he said.
He and his team used a data set including 853 Scottish participants who were given an intelligence test at age 11 and retested between 2008 and 2010 while in their 70s.
Of those, 262 had learned a language in addition to English, most before age 18, though only 90 were actively using the second language in 2008.
Even taking early intelligence scores into account, people who had learned a second language scored higher on reading, verbal fluency and general intelligence in old age than those who never had.
The relationship was the same for the 65 people who learned their second language after age 18, and seemed to get stronger with third, fourth and fifth languages, according to results published in the Annals of Neurology.
“I think it’s a study that could only have been done really with this cohort, this Scottish group,” said Fergus Craik, a senior scientist at the Rotman Research Institute, which is affiliated with the University of Toronto. “I’m not surprised at the effect, but it’s excellent to have this evidence.”
There’s always a question of which comes first, bilingualism or better brains, said Craik, who was not involved in Bak’s study. People by and large become bilingual not because of interest or intelligence but because they have to.
Learning a second language improves certain mental functions, mostly those connected to the frontal lobe of the brain, Craik said. “It does improve fluid intelligence and ‘executive functioning,’ because you have to control the two languages you know,” he said. “While you communicate in one language, you’ve got to manage and control the other language.”
To read the full article Click Here
- Comments (0)
- QEEG differentiates Alzheimer's Dementia from Cognitively Impaired
- By Jay Gunkelman, Chief Science Officer, QEEG-Diplomate
-
Alzheimer's disease and mildly cognitively impaired amnesia clients are often a difficult clinical differential diagnostic quandary. The qEEG can help solve this dilemma
- Comments (1)


 Subscribe to our Feed via RSS
Subscribe to our Feed via RSS